FDA INDICATIONS
Spectron IR Medical Infrared Imaging System
FDA 510(k) Indications for Use
FDA 510(k) #KO32471
Indications for use: The Spectron IR Clinical Infrared Imaging System is intended for adjunctive diagnostic screening for the detection of breast cancer and other uses such as: peripheral vascular disease, neuromusculoskeletal disorders, extracranial cerebral and facial vascular disease, thyroid gland abnormalities, and various other neoplastic, metabolic and inflammatory conditions.
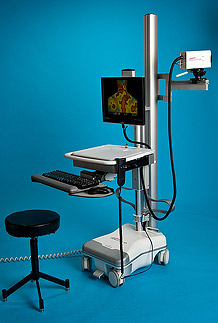
Product FAQs
What are the benefits of having single keystroke software?
The benefits of having single keystroke software include: speed, ease-of-use, and functionality. Why use a mouse to hunt for a drop-down menu, move down the list, click on the function and wait for it to occur. With a single keystroke everything is done and the action occurs.
Does IR Distributions offer any other choices in camera mounts and systems?
IR Distributions Ltd offers a range of solutions from the entry system that include only the camera, computer/laptop and professional software, all the way up to our hospital grade Pro Cart and Wall Mounted systems. We also provide different configurations for mobile systems that include quick and easy mobile conversions.
What is the warranty for the Spectron IR System?
We provide a full one year parts and labor warranty for the Spectron IR system. We additionally provide superior customer service with a well trained staff that includes certified thermography technicians.
